Trial oyster strip spawning at Point Whitney
Today, Steven and I went to Point Whitney to trial strip spawning and fertilization for the Roberts Lab USDA oyster project.
We first sexed two bags of oysters under the dissecting scope. In the first bag, we had 3 males and 3 females with ripe gametes and in the second bag, we only had 1 ripe female and ~10 males. We did fertilization trials with the first batch.
Here is an image of eggs from a female:
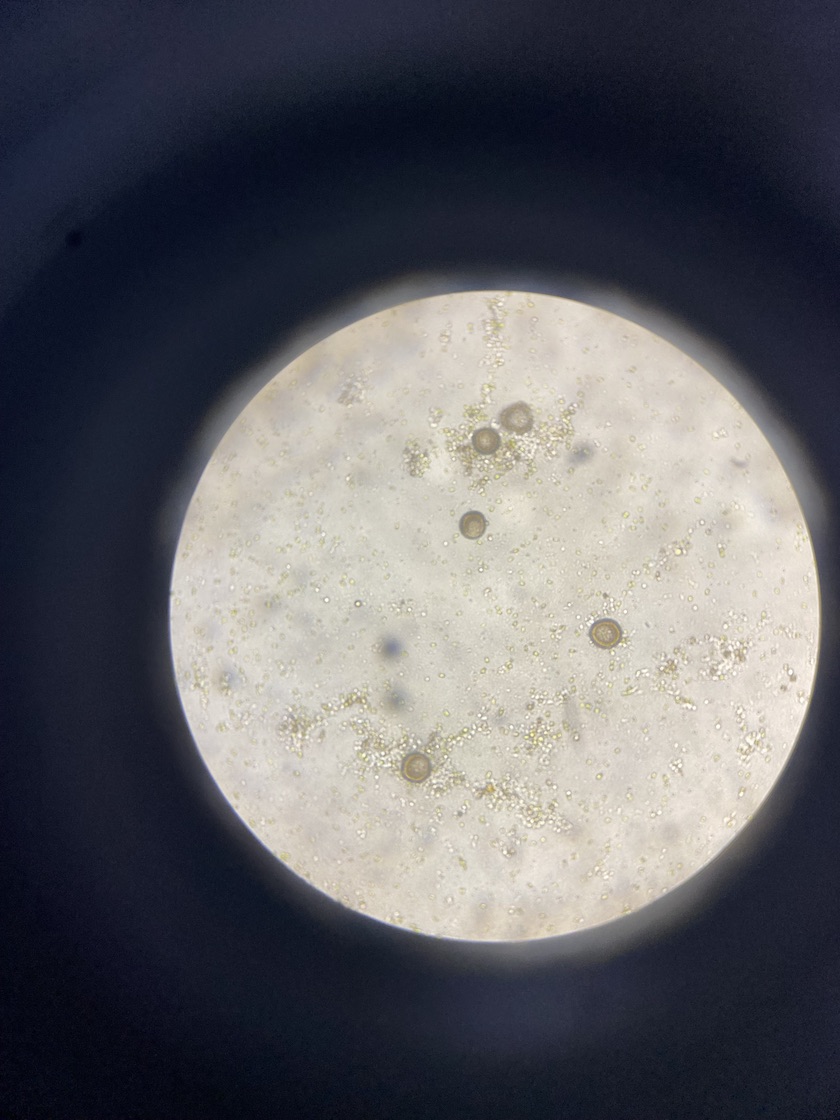
We noticed that some eggs were more tear drop shaped and others had a distinct inner and outer layer. We showed the eggs to Matt at the hatchery and he said the round shape occurs after the eggs hydrate when removed from the adult tissue. I have included some images of egg and sperm below.

We then took the tissue from all the females and all the males and pooled each sex in a plastic bag. We gently mixed the tissue in the bag and then passed the tissue through seives to isolate egg and sperm. To isolate the eggs, we pushed the tissue through the 240 µm mesh onto a 40 µm mesh. The eggs were then rinsed off the 40 µm mesh into a bucket. We noticed that some of the eggs passed through the 40 µm filter, so we passed the eggs again through a 20 µm filter, which captured the remaining eggs. We wondered if the size difference in eggs relates to viability. Perhaps the smaller eggs were not fully developed at this point.

We then isolated the sperm by mixing the male tissue and pushing it through a 20 µm filter. The sperm passed through the filter into a pool below.
After obtaining pools of egg and sperm we did a rough dilution series to look at the ratio of egg to sperm. We did a 1:1, 1:5, and 1:10 ratio of our concentrated egg and sperm pools. We then did an approximate count of how many sperm were in direct proximity to the eggs.
In the 1:1 mix, there were about 40-60 sperm around each egg, in the 1:5 there were 20-30 sperm and in the 1:10 there were about 15-20 sperm. Here is an image of eggs with sperm:
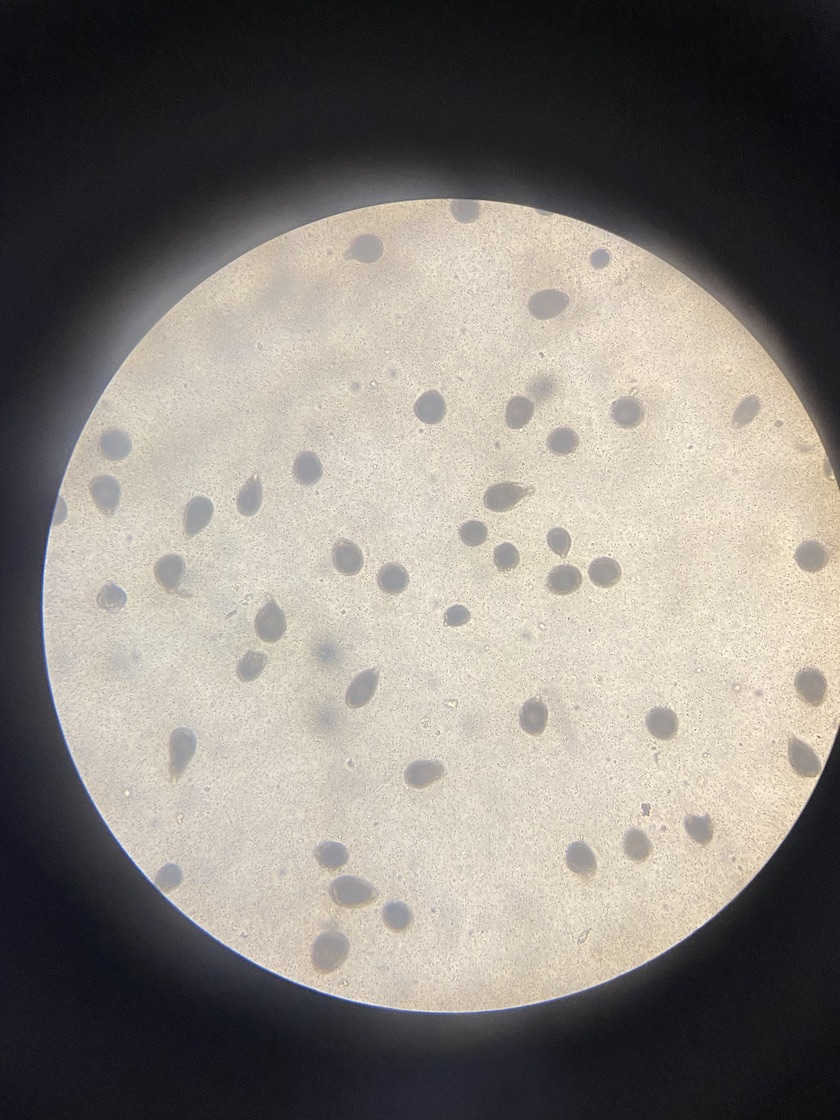
After mixing, we noticed that there was no sperm movement, even though we saw movement during the sexing process. We are unsure why. Perhaps the viable sperm are larger than 20 µm and we filtered them out. We concluded that this seems unlikely from previous protocols and the size of sperm. Perhaps there was an issue with the temperature or quality of the water. Perhaps the gametes were not fully developed.
We checked for fertilization after 1-2 h and we did not observe any cleavage. We expect that there will not be fertilization. Regardless, we combined our remaining egg and sperm pools and divided the pool between 3 HUDLs.
The HUDLs were placed in a water bath at 28°C. Steven will check the cultures to see if anything develops.




